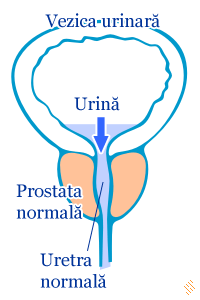
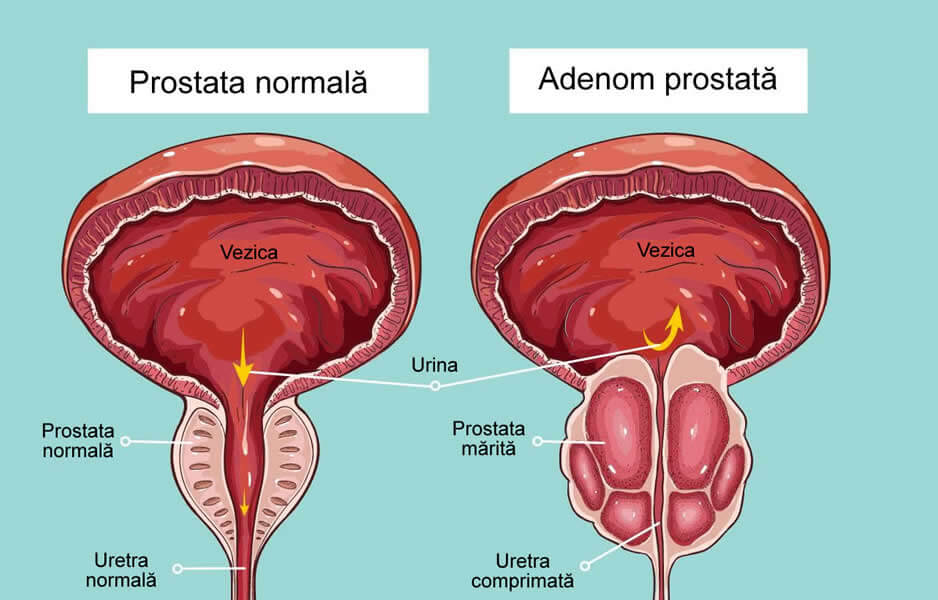
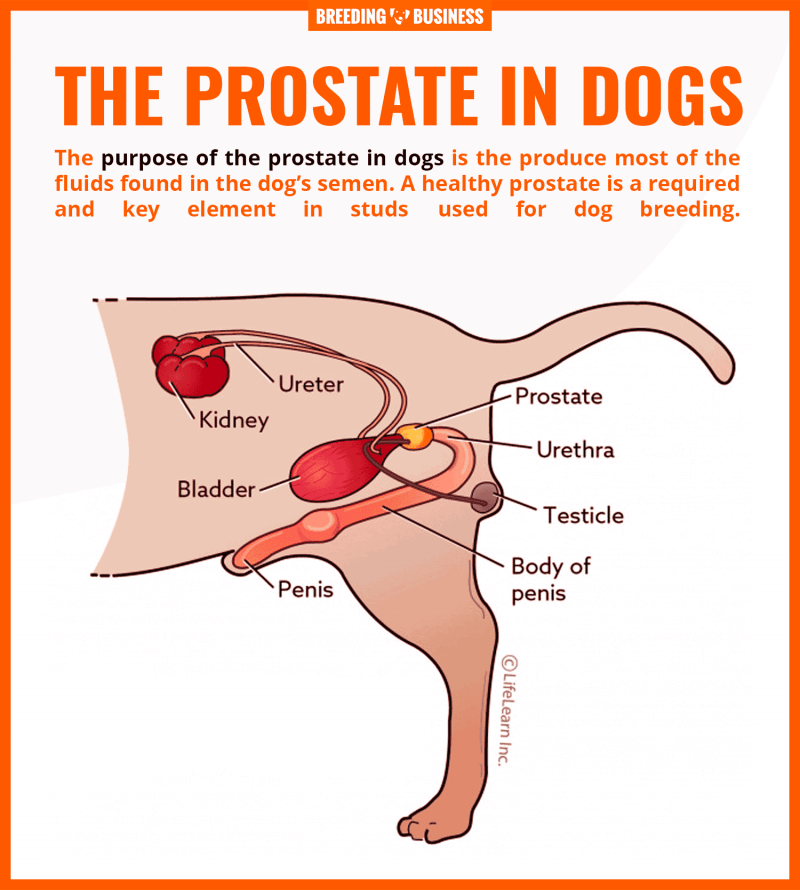
Prostate cancer treatment in dogs. SCIENTIFIC WORKS SERIES C. VETERINARY MEDICINE Volume LXI (2),

Vacancies Leaving no child behind in the fight against cancer The European childhood cancer community has unveiled 6 Key Recommendations to improve access to innovation and medicines for children and adolescents with cancer in Europe. Every 15 minutes, a family in Europe receives the devastating news that their child has cancer.
While every year, 35, new childhood cancer are diagnosed and over 6, young people die from the disease.

In addition, 60 percent ofsurvivors experience long-term adverse side-effects. Although adult cancer therapies are evolving with more innovative medicines reaching Europe, childhood cancer patients are being left behind.

New medicines play a crucial role in improving the quality and length of the lives for many childhood cancer patients but disappointingly this progress has levelled off.
We experience persistent inequalities in access to new and essential medicines for children and adolescents with cancer across Europe. Drug shortages are a major issue with financial barriers seen in lower income countries for standard treatment and more broadly across Europe for newly approved expensive medicines.
On top of the shocking numbers above, there are up to 20 percent differences in survival rates of children with cancer among European regions. Due to their individual rarity, paediatric cancers have seen limited market innovation. Working with this very specific patient group, we take note that there has been a lack of sustainable investment and enabling policies in Europe to enhance innovation for paediatric cancers.
We cannot accept that 10 times less public funding is allocated to childhood cancer research in Europe than in the US. More funding is urgently needed, and we urge Europe to strengthen its position as a leader in childhood cancer research.
The hypodermic preparation of the registered veterinary drug was successfully tested in clinical investigations.
Within this new landscape, we call for a coordinated oversight of the implementation of all paediatric measures within these three major European programmes, with the aim of reaching the principal prostate cancer treatment in dogs of curing more children, curing them better and tackling inequalities.
You might wonder how exactly we can foster progress.
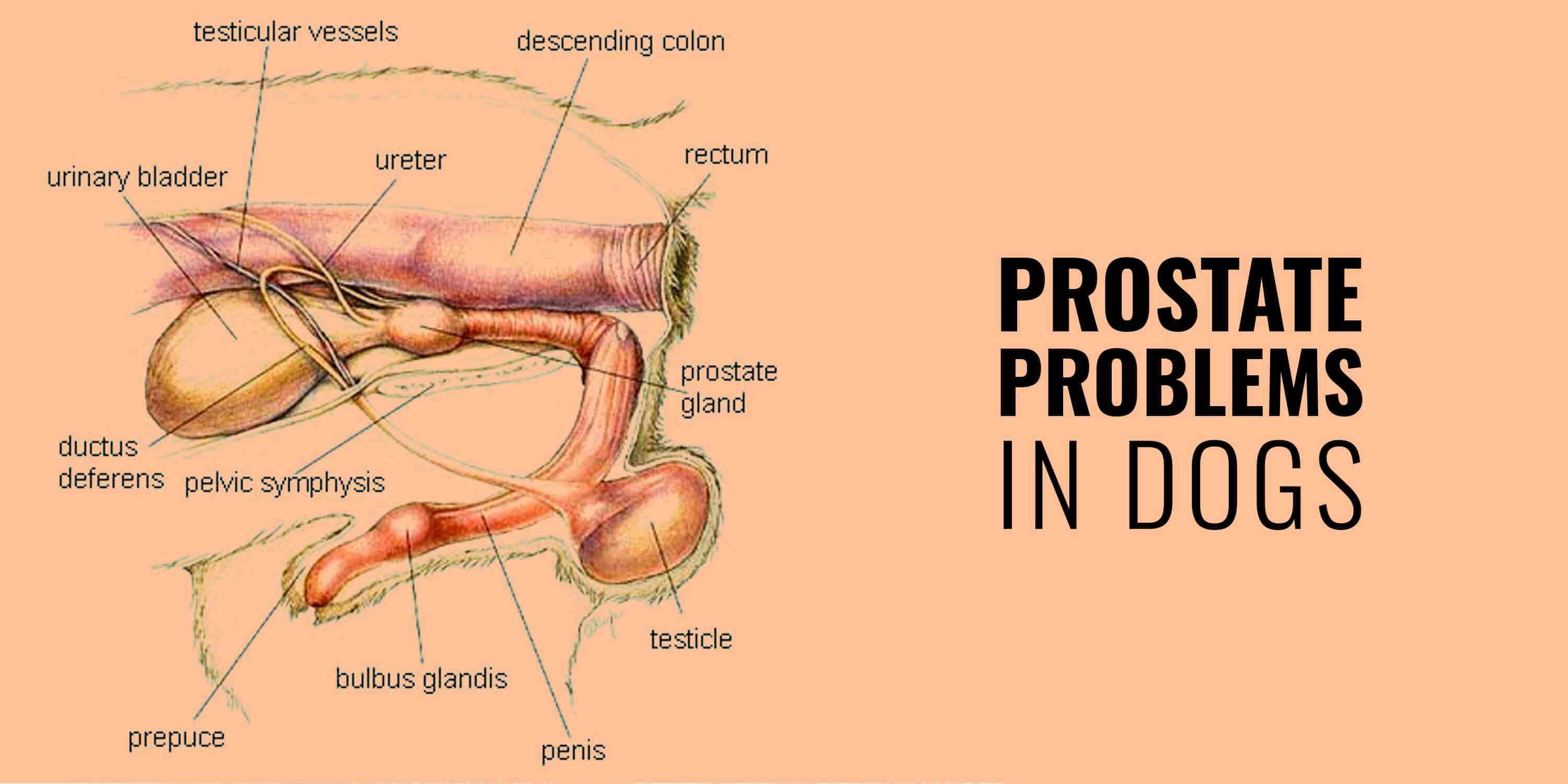
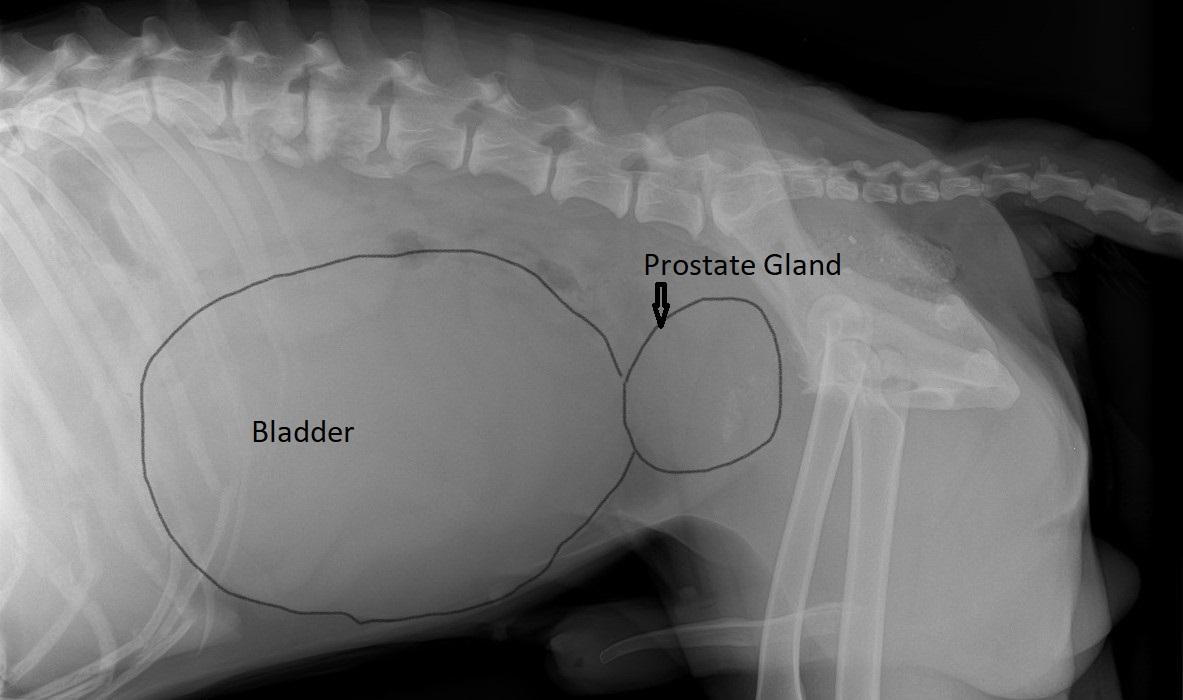
There is no single definitive treatment for dogs with adenocarcinoma of the prostate gland. Nu există nici un tratament unic definitiv pentru câini cu adenocarcinom de prostata.
Why are the existing regulations not accelerating a more enabling environment for research and development of new medicines for paediatric cancers? In the first 10 years since the implementation of the EU Paediatric Regulation, only nine anti-cancer medicines were authorised for a specific paediatric cancer indication, in comparison to over for adult cancers.
The Orphan Regulation was also unable to serve paediatric cancer patients: between and70 ceai prostata fares g74 of anticancer medicines authorised for an orphan indication in adults that had potential relevance to children were not explored in this population. This is simply not good enough as children and adolescents are still dying of cancer across Europe.
For more information: www.